 Hydrogen Snowflakes
Hydrogen Snowflakes
Of all the chemical elements hydrogen is by far the most abundant in the universe. Roughly 3/4 of all the stuff we can see is hydrogen, and most of the remaining 1/4 is helium. All the elements heavier than helium, combined, contribute only about 1% of the visible material.
We also see that hydrogen occurs in various forms in different astronomical environments: as a hot plasma, in which the electrons and protons (nuclei of hydrogen atoms) are not bound to each other; as a gas made up of hydrogen atoms; and as a gas made up of H2 molecules. What about solid hydrogen? Certainly it can be made in terrestrial laboratories, but does it occur naturally in the interstellar medium?
The possibility of solid H2 in the interstellar medium was first considered more than 40 years ago, in connection with interstellar dust particles. The main proponents were Fred Hoyle, Chandra Wickramasinghe and Vince Reddish - all at the Institute of Theoretical Astronomy in Cambridge. The idea was discarded in the late 1960's when it was realised that any particle of solid H2 in the interstellar gas shouldn't last long: the solid is extremely volatile and the sublimation rate is therefore expected to be very high.
It now appears that astronomers were too hasty when we rejected the idea of solid H2 in the interstellar medium. The calculations of how long solid H2 could live for were based on laboratory data for the pure solid, whereas in astrophysical environments the surface of any dust grain acquires electrical charges. These charges cause polarisation of the H2 molecules at the surface, and that binds them more strongly to the rest of the solid, decreasing the sublimation rate. It turns out that this effect is very large, lowering the sublimation rate by a factor of more than 10100 (that number is 1 with a hundred zeros after it) at temperatures below 4 degrees Kelvin. In other words: hydrogen snowflakes live much longer than we thought.
A key factor in the stabilising effect of electrical charges is that solid H2 grains charge up in a manner which is almost unique amongst solids. Rather than acquiring predominantly electrons (which are negatively charged), or positively-charged ions, solid H2 retains both. The positive ions lodge inside the solid matrix and the electrons stick to the surface, outside the solid. In this way H2 snowflakes can be charge-neutral overall, yet have large interior electric fields.
Ironically the reason for this strange charging behaviour, which can stop solid H2 sublimating, is the same as the reason for the extreme volatility of the pure material. The individual molecules have "paired" electrons, so they have no possibility of making any further chemical bonds - the solid is therefore held together only by feeble "dispersion" ("Van der Waals") forces. The tight pairing of the electrons inside each molecule also means that there is "no room" in the solid for any further electrons. Consequently any electrons which collide with the surface become stuck outside, unable to reach the positive ions which are inside, but attracted to them.
So if it's possible for particles of solid H2 to survive in space, perhaps hydrogen snowflakes make up the interstellar dust which we see causing optical extinction and infrared emission? These phenomena are ubiquitous in astrophysics; examples are shown in the images below.
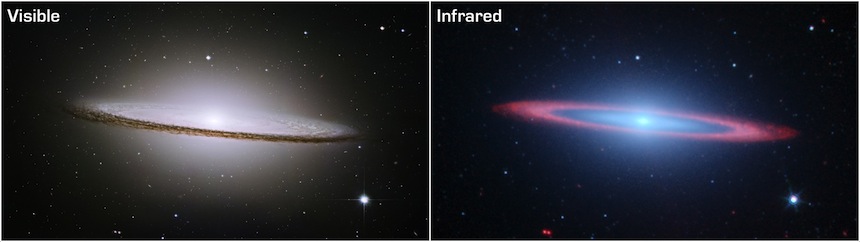 Optical and mid-infrared images of the "Sombrero" galaxy, from the Hubble and Spitzer Space Telescopes (images courtesy NASA/ESA, R. Kennicutt and the SINGS team). In the optical image the dust can be seen as a thin band of extinction running across the galaxy. In the infrared image the dust appears luminous as it re-emits energy that it has absorbed from its environment.
Optical and mid-infrared images of the "Sombrero" galaxy, from the Hubble and Spitzer Space Telescopes (images courtesy NASA/ESA, R. Kennicutt and the SINGS team). In the optical image the dust can be seen as a thin band of extinction running across the galaxy. In the infrared image the dust appears luminous as it re-emits energy that it has absorbed from its environment.
We are now trying to understand how much of the interstellar dust might be made of solid hydrogen. There are various aspects which need to be explored. One thing we have already looked at is the infrared spectral properties - i.e. the precise infrared colours of the dust. For hydrogen snowflakes, the infrared spectra are expected to be mainly due to impurities in the solid, caused by ionisation. The particular impurities which are expected to dominate are the H6+ and (HD)3+ molecular ions. Calculations of their vibrational properties have shown a striking match to the pattern of infrared spectral lines observed from the interstellar medium (see the section on Hydrogen Molecular Ions).
Hydrogen snowflakes play a key role in determining the internal structure of cold, dense molecular clouds, which on this site are termed "Paleons".